What Age Can U Take Birth Control
Why is this important?
Over the past four decades, there has been a dramatic increase in the number of people giving birth at age 35 and older. In the United States (U.S.), this increase started in the mid-1970s and has continued to steadily rise over time. In 2018, 18% of birthing people were 35 and older, up from 15% in 2013, 11% in 2002 and 8% in 1990 (Martin et al. 2019; Martin et al. 2015; Mathews and Hamilton, 2014; Martin et al. 2003). Meanwhile, the overall U.S. birth rate per 1,000 females aged 15-44, as well as the teen birth rate and the birth rate for people in their twenties, are at record lows (Martin et al. 2019).
There has also been an increase in the number of first babies born to people in the U.S. who are 35 or older, from 7.4% in 2000, to 9.1% in 2014 and 10.8% in 2018 (Mathews and Hamilton, 2016; Martin et al. 2019). The average age at first birth is now 26 years of age—a record high for the U.S.! The average age at first birth has increased for all race and Hispanic groups as well as all states and the District of Columbia (D.C.) from 2000 to 2014.
Figure 1 shows changes in birth rates by age from 1970 to 2018 in the U.S.
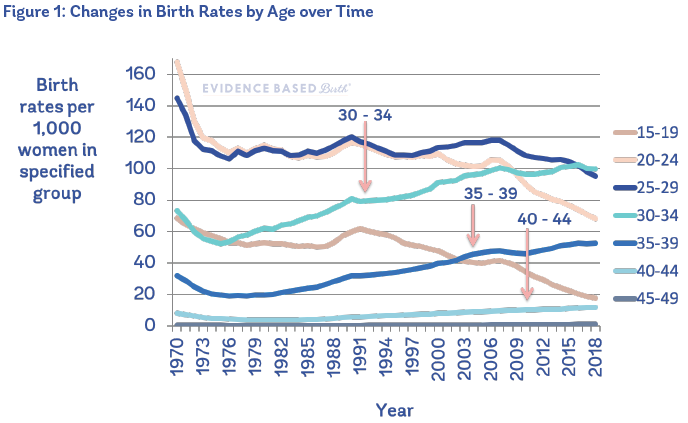
A similar trend is occurring within many high-income countries. For example, in 2013, 20% of births in England and Wales were to people aged 35 years or older and 4% to people 40 years or older compared to 6% and 1% respectively in 1980 (Lean et al. 2017).
Why is parenthood being delayed?
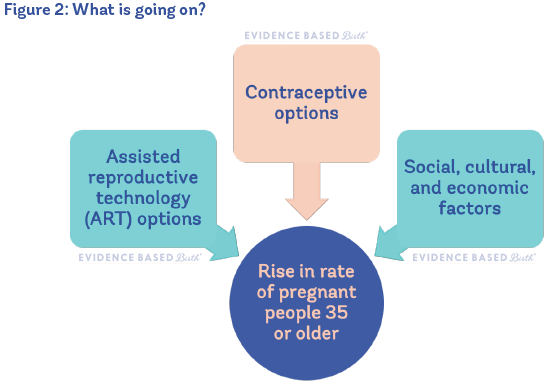
The trend to delay parenthood is happening all around the world. The availability of birth control is partly responsible for more people postponing parenthood. However, birth rates in younger people have also gone down in countries that do not use birth control, so we can't isolate birth control as the main reason. It's thought that, worldwide, there are other social, cultural, and economic factors that play a bigger role than contraception in the increasing age of pregnant people (Mills et al. 2011; Molina-García et al. 2019).
Reasons people delay pregnancy and parenthood include:
- Women are reaching higher educational levels (Mills et al. 2011)
- More women work in male-dominated fields that are not as understanding or supportive of motherhood (Mills et al. 2011)
- Cultural and value shifts have led towards more people not feeling "ready" to have a child yet (Cooke et al. 2010)
- Lack of childcare, low benefit levels, and workplace policies that signal to people that you cannot be both a wage earner and a mother (Mills et al. 2011)
- Economic or housing uncertainty, unemployment, temporary work, or unstable labor markets (Mills et al. 2011)
- Medical reasons (Molina-García et al. 2019)
- Divorce, not knowing the right person to have a child with, and living together before marriage leads some people to delay parenthood (Mills et al. 2011; Molina-García et al. 2019)
Interestingly, while some delay parenthood because of not being in a stable relationship, having a stable partner is actually linked to giving birth to your first child at a later age (Molina-García et al. 2019). University level education, higher income level, and job stability are also linked to giving birth to your first child at a later age.
Chances of getting pregnant at 35 or older
As a person ages, fertility—the chance you will get pregnant or can get your partner pregnant—is reduced. For females, this decline begins slowly in the early thirties and speeds up in the late thirties and forties. If you have been trying to get pregnant for about 12 months and still have not conceived, or if you are 35 and older and have not been able to conceive after trying for 6 months, then it is usually recommended to see a care provider for evaluation for infertility.
Females are born with all of the eggs they will ever have. The decline in fertility with age is due to a decrease in the number of eggs available for fertilization, as well as a decrease in the quality of eggs (i.e. eggs that can produce a genetically typical embryo after fertilization) (Rowe, 2006).
In 1986, researchers carried out a classic study to find out how likely it is for people to get pregnant as they age. They looked at people who were receiving artificial insemination with donor sperm. This study design was important because some people argue that lowered fertility is related to a decrease in the amount of sex people have as they age. However, with this study, there is no way that frequency of sex could explain findings, since the male partners were sterile (Schwartz and Mayaux, 1982).
Participants received artificial insemination once a month for up to one year, or until they became pregnant. Most (74%) of those under the age of 31 were pregnant within one year, but that number went down to 62% of people between 31 and 34, and 54% of people aged 35 and older.
According to the American Society for Reproductive Medicine, the "best reproductive years" are in your twenties. By the age of 30, a healthy, fertile female has a 20% chance of getting pregnant each month of trying to conceive. By age 40, someone has about a 5% chance of getting pregnant each month. In addition to advancing age, sexually transmitted infections, smoking, and having a body mass index (BMI) under 18.5 or above 25 can lead to decreasing fertility.
Male fertility declines with age as well. Researchers have found strong evidence that as males age, they can expect a decline in sperm counts, semen volume, sperm motility, and the number of normal sperm. Older females with older male partners who are trying to get pregnant may experience the combination of female and male age-related fertility decline (Johnson et al. 2015).
You can maximize your chances of getting pregnant at any age by charting your menstrual cycle (either on paper or with an app) to identify your most fertile days. Tracking your fertility signs at home (primarily waking temperature, cervical fluid, and cervical position) can also help to reveal causes of infertility (for example, waking temps can show whether you are ovulating at all). For more info, visit Taking Charge of Your Fertility.
Assisted Reproductive Technology (ART)
One consequence of delayed childbearing is an increase in the use of assisted reproductive technology (ART), which involves the handling of eggs, sperm, or embryos for the purpose of establishing a pregnancy (Zegers-Hochschild et al. 2009). Today, the U.S. Centers for Disease Control and Prevention (CDC) estimates that 2% of all infants born in the U.S. every year are conceived using ART. Advances in ART are a reason why some people can get pregnant after age 35 and especially after age 40.
People trying to become pregnant may be advised to start acupuncture three months before starting ART procedures, or to start acupuncture alongside ART. However, the research evidence on acupuncture as an alternative or complementary treatment for infertility is currently mixed (Yogasundram et al. 2021; Lee et al. 2021).
One ART option that helps some people become parents is the use of third-party reproduction. Third-party reproduction refers to the use of eggs, sperm, or embryos that have been donated by a third person to help an infertile individual or couple give birth to a child. The first successful pregnancies with donor eggs occurred in 1983, and the first successful pregnancy using previously frozen eggs occurred in 1984 (SCRC, 2016). The process of freezing of sperm, eggs, and embryos is called cryopreservation (PBS, 2006).
Artificial insemination involves placing sperm into the uterus or cervix by means other than sexual intercourse. Some single parents-to-be and LGBTQ+ couples use at-home insemination with donor sperm (sometimes known as the "turkey baster" method). Advantages are that at-home insemination is less expensive, easier, and more private than intrauterine insemination (IUI) with a doctor; however, IUI has a higher chance of success.
Intrauterine Insemination (IUI) is an office procedure that places sperm past the cervix and in the uterus around the time of ovulation, which increases the chance that more sperm will meet with the egg. For people with unexplained fertility, the pregnancy rate with IUI is double that over no treatment.
To treat infertility, fertility drugs and IUI are sometimes tried before proceeding to the more costly and invasive in vitro fertilization (IVF) procedure. In vitro fertilization (IVF) is the main type of ART. It involves extracting eggs, fertilizing them in a laboratory, and then transferring the embryos back into the uterus.
Parents, doctors, and research scientists have been working together to overcome infertility since the late 1880s (SCRC, 2016). As scientists got closer to success, a 1969 Harris poll showed that most Americans believed in vitro fertilization was "against God's will" (PBS, 2006). But by 1978, the year of the first "test tube baby," another Harris poll found that most Americans supported IVF and would be willing to try it under the right circumstances.
State laws concerning insurance coverage for ART vary widely across the U.S. A map of the 19 states that have infertility insurance coverage laws can be viewed at the National Infertility Association's website here. According to the American Society for Reproductive Medicine (ASRM), the average cost of one IVF cycle in the U.S. is $12,400. However, this estimate does not include the cost of medications, cryopreservation, and other procedures that are usually necessary to complete one cycle—and most people need more than one cycle. In a large study from the United Kingdom that included 157,974 infertile women with an average age of 35, the authors found that six cycles of IVF led to a live birth rate of 65.3%. However, when they specifically looked at women age 40-42, they observed a live birth rate of 31.5% with six IVF cycles (Smith et al., 2015).
In addition to being cost-prohibitive for many people, there are significant racial disparities in access to IVF. Black and Hispanic people are less likely than white people to access ART, and also less likely to have a successful IVF cycle (Jain, 2020).
In the landmark book, "Killing the Black Body: Race, Reproduction, and the Meaning of Liberty," University of Pennsylvania Professor Dorothy E. Roberts, JD lays out the history of racism and its effects on reproduction in the U.S. In Chapter 6, Roberts describes how ART evolved as an industry designed for white, affluent Americans to have children who are genetically related to them, even though Black people are twice as likely to suffer from infertility. Roberts describes how the disparities in use of IVF "stem from a complex interplay of financial barriers, cultural preferences, and more deliberate professional manipulation." She goes on to contrast the popularity of ART measures for white couples with the long, ongoing history of government-funded programs to limit the fertility of Black women:
"The monumental effort, expense, and technological invention that goes into the new reproduction marks the children produced as especially valuable. It proclaims the unmistakable message that white children merit the spending of billions of dollars toward their creation. Black children, on the other hand, are the object of welfare reform measures designed to discourage poor women's procreation."
~Dorothy E. Roberts, Killing the Black Body
So, in summary, social, cultural, and economic factors; contraceptive options; and ART options are shaping the trend of pregnancies in parents at age 35 and older. Pregnancies with the use of ART tend to be among white, wealthy, heterosexual couples. Meanwhile, Black, Indigenous, and People of Color (BIPOC); those with limited financial means; and LGBTQ+ individuals face more barriers trying to build families with ART.
Figure 2 shows reasons for the rise in pregnant people 35 or older.
Terms
Advanced maternal age (AMA) is usually defined as being 35 or older at the time of giving birth. Since the 1950s and possibly earlier, the age thresholds of 35 and 40 have been used by researchers to label pregnant people as being advanced maternal age.
In the 1970s, age 35 became the cut-off for starting genetic counseling for most people (Berkowitz et al. 2006). At that time, it was thought that the chance of a genetic condition was greater than the risk of pregnancy loss as a result of amniocenteses (an invasive procedure for genetic testing) by the age of 35. Today that risk/benefit calculation is outdated, but age 35 is still widely considered the threshold for advanced maternal age. It's important to understand, however, that age-related concerns rise gradually, not all at once at age 35 (Correa-de-Araujo & Yoon, 2021; Fretts, 2021). The effects of increasing reproductive age exist along a continuum—there is no evidence of a sudden leap in risk between age 34 and age 35.
In addition to the term AMA, people who are 35 or older and pregnant for the first time have been referred to as an elderly primigravida or elderly primipara. Primigravida means a person who is "pregnant for the first time." Primipara means a person who is "giving birth for the first time." Nulliparous is another term that you may see, and this refers to a person who has not yet carried a pregnancy beyond 20 weeks.
Language is so powerful! The terms we use can be empowering and validating, or they can be shaming and demeaning. For example, the label geriatric pregnancy is an outdated and offensive term that is still sometimes used to describe people giving birth at age 35 or older. Because terms such as "elderly" and "advanced maternal age" may be seen as disempowering to some people, for the rest of this article we will simply refer to pregnant and birthing people in this category as being "35 or older."
What are the risks of being pregnant at an older age?
Genetic Conditions
Certain genetic conditions are more common in pregnancies of older people.
One possibility is that the embryo will have Down syndrome, which happens when there is an extra copy of Chromosome 21. According to the CDC, about one in every 700 babies born in the U.S. is born with Down syndrome, making Down syndrome the most common chromosomal condition in liveborn infants. The rate of having a baby with Down syndrome increases with the pregnant person's age—this has been seen in large studies of pregnant people, as well as in studies with embryos conceived with IVF (Haddow et al. 2009). It's actually the egg's age and not the pregnant person's age that determines chances of Down syndrome. So when older people use younger eggs, they lower their chances of having an embryo with Down syndrome.
These are the age-related rates of an embryo having Down syndrome at 10 weeks of pregnancy:
- 1 in 1,064 at age 25
- 1 in 686 at age 30
- 1 in 240 at age 35
- 1 in 53 at age 40
- 1 in 19 at age 45
These are the live birth rates of having a baby with Down syndrome at term:
- 1 in 1,340 at age 25
- 1 in 939 at age 30
- 1 in 353 at age 35
- 1 in 85 at age 40
- 1 in 35 at age 45
The rates of having a baby with Down syndrome at term are not as high as the chances at 10 weeks, mostly because these pregnancies have higher rates of miscarriage and stillbirth and won't all reach the term period.
Older age at birth can also increase the chance of other chromosomal conditions. Trisomy 18 (Edward syndrome) is the second most common chromosomal condition at the time of birth (about 1 in 3,000 live births), and trisomy 13 (Patau syndrome) occurs in about 1 in 6,000 live births. The most common sex chromosome condition is 47, XXY (Klinefelter syndrome), which occurs in about 1 in 500 males (ACOG et al., 2020).
In the U.S., ACOG recommends that screening and diagnostic testing for chromosomal conditions be discussed and offered to everyone early in pregnancy regardless of their age or risk factors. However, they say that people should be counseled regarding their specific risks based on their age and their genetic family history (ACOG et al. 2020).
Risk of miscarriage
The rate of spontaneous miscarriage (pregnancy loss before 20 weeks) climbs gradually with age (CDC, 2020). The increased risk of miscarriage in older pregnant people is related to both egg quality and an increase in preexisting medical conditions.
A large Norwegian study recently found that the risk of miscarriage was (Magnus et al. 2019):
- 17% at <20 years
- 11% at 20-24 years
- 10% at 25-29 years
- 11% at 30-34 years
- 17% at 35-39 years
- 33% at 40-44 years
- 57% at >45 years
This is called a J-shaped association because the rates are elevated among teens, low among people 20-34, and then increase again, especially for people in their forties.
Risk of stillbirth
In the U.S., stillbirths are typically defined as pregnancy losses that take place at or after 20 weeks of pregnancy (CDC, 2020). There are two ways of looking at the risk of stillbirth in people who are 35 or older. One way is to look at the absolute risk; this is the actual rate of stillbirth among people of a certain age group. This means you can say something like "Among pregnant people 35-39 years of age, X number of babies out of every 1,000 births are stillborn." For example, one study found the absolute risk of having a stillbirth at ages 35-39 was 0.22%, meaning then that 2.2 pregnant people out of 1,000 in this age group experienced a stillbirth (Kortekaas et al. 2020).
The other way is to look at the relative risk. This means that you compare the risk of stillbirth among older pregnant people to the risk experienced by younger pregnant people. This approach will give us a result like, "Compared to people 18-34 years of age, those aged 35-39 are X% more likely to experience stillbirth."
With relative risk, if a risk is "50% higher," this does not mean that an older person has a 50% chance (1 in 2 chance) of having a stillbirth. For example, if the absolute risk of a stillbirth is 2 per 1,000 in someone aged 35, and someone aged 40 has a risk of 3 per 1,000, then that would be a 50% increase in risk. Similarly, a risk that is "100% higher" does not mean that stillbirth is a certainty. A 100% increase in risk sounds huge, but if the risk began as 1 in 1,000 people, a 100% increase in risk means that 2 out of 1,000 people will be affected.
Huang's 2008 systematic review
In 2008, Huang et al. published a systematic review of all the studies from the previous ten years that looked at the risk of stillbirth with increasing age. They looked at 37 studies, and the overall quality of these studies was good. Participants in these studies gave birth any time between the years 1959 to 2003, so some of the studies took place a long time ago, and some were more recent. Most of the studies were very large, with half having a sample size over 78,000 births per study (Huang et al. 2008).
The results? In Huang's systematic review, researchers in 24 out of 31 studies found that older pregnant people were more likely to have a stillbirth than younger pregnant people.
When they only looked at studies from developed countries, the risk of having a stillbirth was about 1.2 to 2.23 times higher among older people when compared to younger people.
The increased risk of stillbirth with age was not due to other known risk factors for stillbirth.
The Huang review was limited in that most of the studies (29/31) picked an arbitrary age (usually 35 or 40 years) and compared the stillbirth rates between two groups, either above or below the cut-off point. It would have been better if they had also reported how the risk increases year by year so that we could see the trajectory. The results from Huang et al.'s review, lumped into one of two groups, make it seem like there is a drastic increase in risk at age 35. However, as we mentioned before, the risk might instead rise gradually each year.
In spite of the fact that each of the studies was so different and took place in such different time periods, Huang et al. found that, overall, the study results were consistent in that almost every one found an increased risk of stillbirth in older pregnant people.
But what is the actual risk of stillbirth in pregnant people who are 35 or older?
So we know that the risk of stillbirth is higher in people who are 35 or older. But what are the actual numbers? If someone is 35 or older, what is their chance of having a stillbirth?
In 2000, Jolly et al. looked at the medical records of 358,120 people who gave birth at 18 hospitals in the United Kingdom (U.K.) between the years 1988 and 1997 (Jolly et al. 2000). They found that pregnant people aged 35 and over were at increased risk of stillbirth even after considering body mass index, ethnic group, medical problems, and whether or not the mother was giving birth to her first child. However, the authors did not specify their definition of stillbirth. This means that the babies could have been stillborn starting at any gestational age after 20 to 28 weeks.
The researchers in the United Kingdom found:
- People aged 18 to 34 had a stillbirth rate of 4.7 per 1,000, or 0.47%
- People between 35 and 40 years old had a stillbirth rate of 6.1 per 1,000, or 0.61%
- People 40 and older had a stillbirth rate of 8.1 per 1,000, or 0.81%
A larger and more recent study from the Netherlands included over 1.6 million people who gave birth to a single baby at term from 1999 to 2010 (Kortekaas et al. 2020). They excluded people with pregnancies complicated by congenital anomalies, hypertensive disorders, or diabetes.
The researchers examined many adverse pregnancy outcomes by maternal age, including stillbirth (occurring between 37 weeks + 0 days to 42 weeks + 6 days).
The researchers in the Netherlands found:
- People aged 18 to 34 had a stillbirth rate of 1.7 per 1,000, or 0.17%
- People between 35 and 39 years old had a stillbirth rate of 2.2 per 1,000, or 0.22%
- People 40 and older had a stillbirth rate of 3.0 per 1,000, or 0.30%
These stillbirth rates are lower than those in the Jolly et al. study, most likely because they excluded higher-risk pregnancies and late preterm losses. Also, these pregnancies occurred more recently and may have benefited from advances in care.
Do the risks increase for EVERYONE, or just for people giving birth for the first time at or past 35?
In 2006, a large observational study by Reddy et al. used U.S. birth certificate data from 2001 to 2002 to look at maternal age, stillbirth rates, and whether or not the risk was higher for people having their first baby. They included information from 5.5 million pregnancies in which people were pregnant with a single baby with no birth defects. Stillbirth was defined as fetal death occurring at 20 weeks of pregnancy or longer (Reddy et al. 2006).
As you can see in the table below, pregnancy at age 35 or older is linked to an increased risk of stillbirth in both first-time parents and people who have given birth before. However, the rates of stillbirth are higher for everyone who is having their first baby compared to subsequent babies, no matter the person's age. People who are having a baby at the age of 40 or older and have given birth before, actually have a lower stillbirth risk than those giving birth for the first time who are under the age of 35. The highest rates of stillbirth are with older first-time mothers, though the absolute risk is still low.
Table 1 shows stillbirth rates for people of different ages giving birth for the first time.

For pregnant people 35 or older, does the risk of stillbirth increase as they get closer to their due date?
Many people 35 or older are told they should be induced as they get closer to their due dates due to the risk of stillbirth. Because of this, it's important for us to know the rates of stillbirth towards the end of pregnancy—not just the overall rate of stillbirth. *The evidence for induction for those age 35 and older is reviewed later in this article.
In 2006, Reddy et al. looked at gestational age and risk of stillbirth among pregnant people in the U.S. This was the same study listed above, where they looked at 5.5 million birth certificates from singleton pregnancies without birth defects.
When pregnant people of all ages were grouped together, the highest risk of stillbirth occurred around 41 weeks, followed by 20-23 weeks. But when they separated people by age, those 35 and older had the largest increase in risk of stillbirth starting at around 39 weeks. Between 39 and 40 weeks, about 1 out of 1,000 pregnant people younger than 35 had a stillbirth, compared to 1.4 out of 1,000 among people 35 to 39, and 2 out of 1,000 among people 40 and older.
The figure below shows the risk of stillbirth going up with gestational age among three groups: people less than 35, those aged 35 to 39, and those 40 or older. You can see that for pregnant people 40 and older, the largest jump in risk happened between 38 and 39 weeks and then the risk continued to increase.
Figure 3 shows details on gestational age and risk of stillbirth in the U.S. (2001-2002).

In 2010, Haavaldsen et al. published a paper looking at the risk of stillbirth by gestational age among younger and older pregnant people (Haavaldsen et al. 2010). The researchers looked at more than 2.1 million pregnancies in the Medical Birth Registry of Norway between the years 1967 and 2006. To be included in the study, people had to give birth to a child at 16 weeks gestation or later. They found that, overall, people 35 and older had higher rates of stillbirth throughout pregnancy as compared to younger people, but the risk was higher at 40, 41, 42, and 43 weeks. The table below shows the stillbirth rates from the entire time period, including the 1960s and 1970s. Keep reading below this table to learn the more current stillbirth rates!
Table 2 shows the number of stillbirths per 1,000 ongoing pregnancies in Norway (using data from years 1967 to 2006).
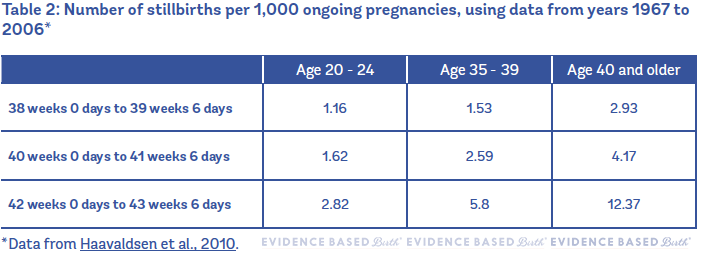
However, you have to remember that this table included people who gave birth as long as 50+ years ago.
When the authors broke down the data into two separate time periods, they found very different results! Haavaldsen et al. divided their data set into two sections: 1) studies from 1967 to 1986 and 2) studies from 1987 to 2006. In the more recent studies, there is still a higher relative risk of stillbirth at the end of pregnancy among people age 35 and older, but the risk is much, much lower than it used to be. For example, it used to be that the risk of stillbirth at 42 weeks in people 40 years and older was 12.37 per 1,000… and now it's 2.64 per 1,000.
Table 3 shows the number of stillbirths per 1,000 ongoing pregnancies in Norway (using data from years 1987 to 2006).
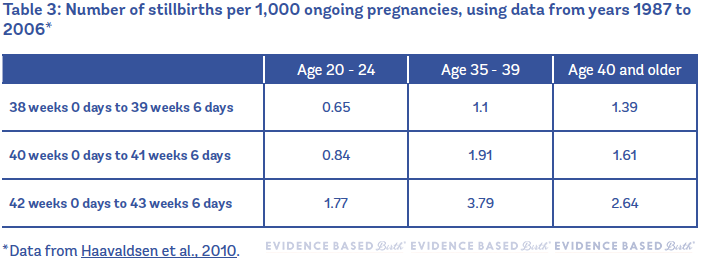
The takeaway point from this study is that it's really important to have current information about stillbirth rates. So much about maternity care has changed in the past few decades! Older data do not give us an accurate picture of the risks of being pregnant at age 35 or older.
What if someone is healthy and over the age of 35? Do they still have a higher risk of stillbirth?
Reddy et al. (2006) also looked at this question in their study of 5.5 million U.S. birth certificates. When they compared healthy people (no diabetes, no high blood pressure, no preeclampsia, no kidney/heart/ lung disease) to the overall sample, they found that the healthy people had a lower risk of stillbirth, but the risk still went up with age.
In other words, being healthy lowers your risk of stillbirth, but if you are 35 or older, your risk as a healthy older person is still higher when compared to a healthy younger person.
Figure 4 shows the overall sample vs. healthy people only.
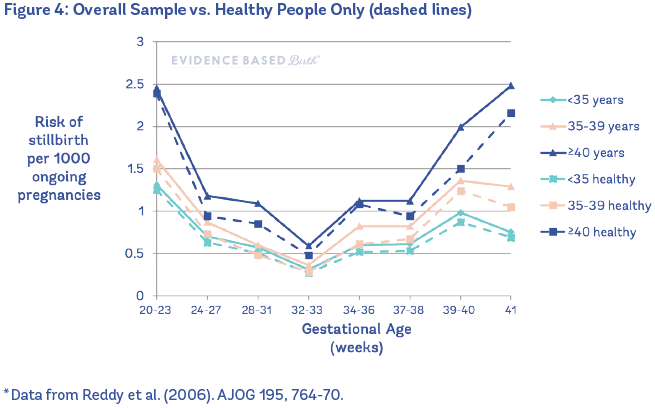
In 2017, a large systematic review and meta-analysis of the effects of the pregnant person's age on outcomes also found that the increased risk of stillbirth with age was not fully explained by maternal medical conditions (Lean et al. 2017).
The authors analyzed 44 observational studies with nearly 45 million births, including over 185,000 stillbirths. The majority of studies were conducted in high-income countries and judged to be low risk of bias. They found a significantly increased risk of stillbirth with pregnancy at age 35 and older, independent of maternal health status. Overall, about 5% of total stillbirths in the study population were attributed to pregnancy at age 35 or older.
Why does the risk of stillbirth increase with maternal age?
Researchers don't know why there is an increased risk of stillbirth in pregnant people as they age. The relationship is complicated and not completely understood.
Some think that the increased risk of stillbirth may be related to other health problems that are more likely to occur with aging, such as high blood pressure, gestational diabetes, high BMI, and other chronic medical problems. However, as we already talked about, it looks like medical problems do not fully explain the increased risk of stillbirth—because even when we remove these risk factors, healthy people 35 and older still have an increased risk of stillbirth (Huang et al. 2008; Reddy et al. 2006; Lean et al. 2017).
Lean et al. (2017) proposed that older people are at greater risk of placental problems (pathology or dysfunction), and this could be increasing their risk of stillbirth. In a meta-analysis, they looked at 12 studies on fetal growth restriction (defined as birthweight below the 5th percentile for gestational age). They found that older maternal age was linked to an increased risk of having this complication. The risk of fetal growth restriction was only significantly increased over the age of 40.
In addition to fetal growth restriction, they also found that older age was linked to increased risk of placental abruption and preeclampsia. Since these are all conditions related to placental pathology/dysfunction and to stillbirth, the authors concluded that placental factors are "a logical avenue to explore," especially in pregnant people over the age of 40.
Another possibility is that advanced paternal age could be contributing to the increased risk of stillbirth in older pregnant people (Lean et al. 2017). Older paternal age is often coupled with older maternal age. The effect of older fathers on stillbirth has received very little attention, but at least one study has found an increase in the rate of stillbirth with paternal age 40 and over, independent of the pregnant person's age (Alio et al. 2012). In addition, a systematic review and meta-analysis of 10 observational studies found that paternal age 40 and over was significantly linked to an increased risk of spontaneous miscarriage, taking into account the pregnant person's age (du Fossé et al. 2020). However, the effect was less pronounced than seen with older maternal age.
Again, at this time, there is a pressing need for further study, because the reason for the increased risk of stillbirth in these families is unknown.
Both younger age (less than 15 years) and older age (greater than 35 years) are independent risk factors for stillbirth. In addition to older age, many other factors are known to affect the risk of stillbirth, including being pregnant with your first baby, a body mass index (BMI) ≥ 30, preexisting diabetes, chronic high blood pressure, smoking, alcohol use, having a pregnancy using assisted reproductive technology, being pregnant with multiples, male fetal sex, unmarried status, placenta previa, and a history of previous stillbirth (ACOG/SMFM, 2020). Discussing your individual risk factors with a care provider can be helpful.
Importantly, Black birthing people are 1.5 to 2 times more likely than white birthing people to have a stillbirth at every week of pregnancy (Muglu et al. 2019). Racial health disparities are due to racism in all of its forms, including the effects of prejudice and institutional/systemic racism (Williams and Mohammed, 2013; Bailey et al. 2017). Evidence-based solutions to mitigate racial disparities in pregnancy outcomes include doula support and midwife-led models of care (Bohren et al. 2017; Kozhimannil et al. 2016; Thomas et al. 2017; Sandall et al. 2016).
For example, the innovative JJ Way® Maternity Care Model, founded by Black midwife Jennie Joseph, effectively eliminates racial disparities in preterm birth outcomes (Josephs and Brown, 2017). The comprehensive Easy Access Clinic ensures everyone receives care—no one is turned away. Clinicians develop relationships with clients and bond through mutual respect and education. There ARE solutions to racial disparities! Black birth workers know the solutions and they are saving lives.
Are there any other risks?
Besides stillbirth, researchers have found increases in a number of other childbirth risks in people 35 and older. In general, rates of complications go up as maternal age increases.
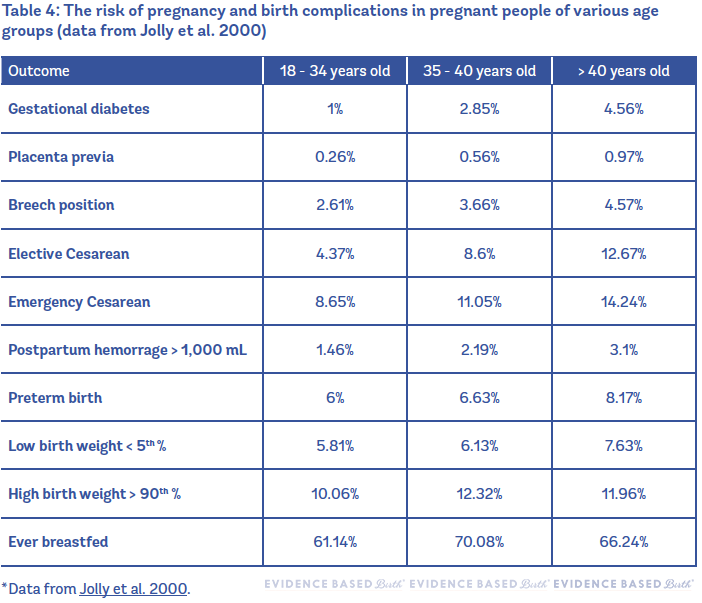
One large study using data from 385,120 singleton pregnancies in the U.K. (between the years 1988 and 1997) compared the rates of pregnancy complications between people aged 18 to 34, 35 to 40, and over 40 (Jolly et al. 2000). Most risks were found to increase with age. Encouragingly, breastfeeding rates were higher in people 35 and older than in the younger group.
Table 4 compares the risk of pregnancy and birth complications in pregnant people of various age groups.
Another large, high-quality study used data from the Swedish Medical Birth Register collected between 1987 and 2001 (Jacobsson et al. 2004). In this study, the authors looked at 909,228 births and compared outcomes between groups of people aged 20 to 29, 40 to 44, and 45 and older. They did not look at the 30-to-39 age group. This study found similar results as the Jolly study (see Table 4).
Importantly, Jacobsson et al. 2004 found that the risk of the mother dying (maternal mortality) increased with age. During the 15 years of the study, there were 21 maternal deaths in the more than 900,000 births. When maternal death rates were separated out by age group, they found a maternal death rate of 1.4 per 100,000 for people aged 20 to 29, 22 per 100,000 for people aged 40 to 44, and 166 per 100,000 for people 45 and older. Unfortunately, the researchers did not offer any explanation or describe the causes of these deaths, which primarily occurred in the 1980s-1990s.
We also have recent data from the very large study of over 1.6 million people who gave birth to a single baby at term in the Netherlands (Kortekaas et al. 2020). As mentioned above, they excluded pregnancies complicated by congenital anomalies, hypertensive disorders, or diabetes.
After considering gestational age, onset of labor (spontaneous versus induction or elective Cesarean), and whether or not this was the pregnant person's first birth, the researchers found significantly increasing rates of poor perinatal and maternal outcomes at 35 years and over. They looked at composite outcomes, meaning several outcomes combined into a single measure. The composite adverse perinatal outcome included stillbirth, newborn death, meconium aspiration syndrome, 5-minute Apgar score <7, NICU admittance and sepsis. The composite adverse maternal outcome included maternal death, placental abruption and postpartum hemorrhage (bleeding) of >1,000 mL.
- People aged 18 to 34 had a composite adverse perinatal outcome of 1.6% and a composite adverse maternal outcome of 4.6%
- People between 35 and 39 years old had a composite adverse perinatal outcome of 1.7% and a composite adverse maternal outcome of 5.0%
- People 40 and older had a composite adverse perinatal outcome of 2.2% and a composite adverse maternal outcome of 5.2%
Some researchers have expressed concerns that most of the research on birth outcomes at advanced maternal age focuses on fetal and infant outcomes, and not enough attention is given to risks for the pregnant person. To examine the risk of maternal death and severe complications, a recent study looked back at over 800,000 births to single babies in Washington State between 2003 and 2013 (Lisonkova et al. 2017). All live births and stillbirths to pregnant people aged 15-60 years were included in the dataset.
Despite the large sample size, the study was not large enough to examine the link between maternal age and maternal death. Very few maternal deaths occurred (less than 1 per 10,000 births in each age group). So the authors looked at a composite outcome of maternal death or severe complications. They defined "severe maternal morbidity" broadly, including a long list of conditions such as hemorrhage with transfusion, sepsis, complications of anesthesia, and ICU admission.
After taking other factors into account (race, marital status, BMI, drug use, smoking, number of previous births, maternal education, type of health insurance, year of childbirth, and fetal sex) they found that the rate of potentially life-threatening complications significantly increased with maternal age. The rate of maternal death or severe complications was around 21 per 1,000 births at 15-19 years; 16 per 1,000 at 20-24; 14 per 1,000 at 25-34; 18 per 1,000 at 35-39; 23 per 1,000 at 40-44; and 36 per 1,000 at 45 and older. This is another example of a J-shaped association.
These rates seem high because the authors counted so many conditions as "severe". But it's reassuring to note that the absolute risk of maternal death was low for every age group. Another way to look at these findings is that the vast majority of pregnant people did NOT experience serious medical problems: 979 per 1,000 births at 15-19 years; 984 per 1,000 at 20-24; 986 per 1,000 at 25-34; 982 per 1,000 at 35-39; 977 per 1,000 at 40-44; and 964 per 1,000 at 45 and older.
People who gave birth aged 40 and older had significantly higher rates of some of the most serious but rare outcomes, including kidney failure (4 to 28 per 10,000), shock (4 to 7 per 10,000), acute heart problems (7 to 16 per 10,000), and ICU admission (16 to 80 per 10,000). The authors suggest that people considering delaying childbirth until their forties should be aware of these findings so that they can make a fully informed decision.
A much larger study of over 36 million U.S. births from 2006 to 2015 also focused on severe maternal complications by age (Sheen et al. 2018). After taking other relevant factors into account, people aged 35 and older had significantly higher relative risk of complications compared with people 25-29 years. People aged 45 years and older were at the highest risk for complications during childbirth. For example, Cesarean (63%), preeclampsia (10%), postpartum hemorrhage (5%), gestational diabetes (16%), thrombosis (a clot inside a blood vessel; 6 per 10,000), and hysterectomy (surgical removal of the uterus; 103 per 10,000) were most common among people aged 45 and older.
Research focusing on pregnancy at age 45 and older
Researchers in Switzerland looked back in time to compare pregnancy outcomes between 127 people 45 years and older with around 2,000 people aged 30 at the time of birth (Haslinger et al. 2016).
Pregnant people 45 and older had a higher rate of maternal complications such as high blood pressure during pregnancy (3.9% vs 0.6%), preeclampsia (14.2% vs 3.0%), gestational diabetes (12.6% vs 3.6%), increased need for blood transfusion (3.2% vs 0.7%) and hospitalization >7 days (37.8% vs 15.1%). Infant complications such as prematurity (44.9% vs 16.2%) and low birthweight (11.0% vs 5.6%) were also increased.
The researchers advised that people postponing pregnancy or planning a pregnancy at ≥45 years should be informed about these risks.
A study using data from the UK Obstetric Surveillance System (UKOSS) compared 233 people who gave birth aged 48 years and older to 454 people aged 16 to 46. They found that most of the increased risks with pregnancy ≥48 were explained by having multiples or the use of assisted conception (Fitzpatrick et al. 2017). After they took those factors into account, most pregnancy risks went down, except for gestational diabetes, Cesarean, and ICU admission.
In summary, there are increases in risk of many health problems as a pregnant person ages, even after controlling for other risk factors that increase with age. Some differences are fairly small and can only be seen with large sample sizes. Other risks, such as the risk of Cesarean, increase more dramatically.
How many pregnant people 35 or older are induced or have Cesareans?
In the U.S., the Centers for Disease Control and Prevention's National Vital Statistics Reports include supplemental tables with this information.
Induction rates do not seem to increase with age when you look at U.S. birth certificate data. The overall induction rates from 2018 are: 27.5% (people age 25 to 29), 25.6% (age 30 to 34), 25.0% (age 35 to 39), and 27.1% (age 40 to 54).
Cesarean rates, however, climb steadily with age. Cesarean rates are 30.0% (age 25 to 29), 33.9% (age 30 to 34), 40.1% (age 35 to 39), and 48.0% (age 40 to 54).
The increase in Cesarean rates with older age is consistent in the research, and the pattern is the same whether labor is induced or spontaneous (Bergholt et al. 2020). There are many possible reasons for this, including more medical conditions in older pregnant people. Care provider perception is another variable here that is hard to measure. With interventions like Cesarean, it's possible that perception can be a self-fulfilling prophecy. In the Evidence Based Birth® Signature Article about Suspected Big Babies, we found research showing that the more significant risk was not the big baby itself, but the care provider's perception of a big baby. With people who are 35 or older, the care provider's perception that a person is "high-risk" because they are older might lead to a higher chance of them having an intervention, regardless of the actual need for the intervention. It is not clear what percentage of Cesareans among people 35 and older occur because of an actual health risk or the perception of risk.
Physiological effects of being 35 and older may also impact risk of Cesarean. Older age is an independent risk factor for being diagnosed with slow labor (called labor dystocia) among both first-time and experienced birthing people (Waldenström and Ekéus, 2017). But again, care provider bias about labor progress with people who are 35 and older may lead the care providers to over-diagnose labor dystocia.
What is the evidence for electively inducing labor in people who are 35 or older?
The Fonseca 2020 meta-analysis
The largest meta-analysis on the effects of elective induction at term on rates of Cesarean among pregnant people aged 35 and older was published in 2020 (Fonseca et al. 2020). A meta-analysis pools data from multiple studies into one big study. The researchers included over 81,000 participants from a total of eight studies; six were randomized, controlled trials and two were observational studies.
As we'll discuss below, there has only been one randomized trial (the "35/39 Trial") done to compare elective induction versus expectant management (waiting for labor to start on its own or having a planned birth at a later time) in people aged 35 and older. However, several studies have compared elective induction versus expectant management in both older and younger people (i.e. the ARRIVE trial). So, for the Fonseca meta-analysis, the authors included a subgroup of participants aged 35 and older from many different studies. Everyone had intact membranes and a single, head-down baby at term.
At 39 weeks' gestation, over 26,600 participants aged 35 and older were assigned to elective induction and over 54,500 were assigned to expectant management. Elective induction had no overall effect on rates of Cesarean, vaginal birth with forceps/vacuum, or postpartum hemorrhage. Their study was not large enough to examine stillbirth or newborn outcomes.
An earlier meta-analysis on this topic also found that induction in people 35 years or older had no effect on Cesarean rates (Walker et al. 2016b).
The 35/39 Trial
In 2016, Walker et al. published a randomized, controlled trial in the New England Journal of Medicine (Walker et al. 2016a). During the years 2012 to 2015, about 600 pregnant people from 42 hospitals in the U.K. were randomly assigned (like flipping a coin) to either inducing labor between 39 weeks 0 days and 39 weeks 6 days, or not inducing at 39 weeks and instead waiting up until 41-42 weeks before being induced.
People could participate in the study if they would be 35 years or older on their due date, had never given birth to a live baby, and were pregnant with a single baby in head-first position. People were not included if they had gestational diabetes or were pregnant with the help of in vitro fertilization from donor eggs.
The group that was not induced in the 39th week did not receive any special fetal testing or monitoring before 42 weeks, unless it was the usual practice of the individual physician. The researchers did not tell the doctors what type of induction methods participants should receive—any inductions were done per hospital protocol. Most hospitals tended to use prostaglandin gel to ripen the cervix, followed by Pitocin® and artificial breaking of the waters.
Most of the participants in the 39-week induction group (78%) were actually induced; 20% went into spontaneous labor before their induction and a few people had Cesareans. About half of participants in the expectant management group went into labor on their own (46%), while the other half (49%) were induced further along in pregnancy or had Cesareans. The most common reasons for induction in the expectant management group included: reaching 41 weeks, the water breaking before labor at term, reduced fetal movements, and request of the pregnant person.
The results? There was no difference in Cesarean rates between the induction at 39 weeks group and the not-induced-at-39-weeks groups. The Cesarean rates were about 32-33% in both groups, and the rates went up with the mother's age. There was also no significant difference in the rate of vaginal birth with forceps or vacuum, or complication rates for pregnant people, including placental abruption, cord prolapse, postpartum hemorrhage, shoulder dystocia, need for blood transfusion, or infections. There were no maternal deaths in either group. Both groups had similar birth satisfaction scores.
As far as the results for babies, there were no deaths or stillbirths in either group. There were also no differences between groups in average birth weight, Apgar scores, umbilical cord pH levels, NICU admissions, low blood oxygen, or need for interventions such as tube feeding or oxygen.
This one randomized trial indicates that induction of labor does not improve outcomes or Cesarean rates for people giving birth for the first time at greater than 35 years; however, this study was simply too small to tell if induction can decrease the risk of stillbirth or newborn death. There were about 600 participants, and zero deaths. The authors state that if another study investigates this issue, it would need to be extremely large.
You can learn more about the 35/39 trial at their website here.
The Knight Retrospective Study
In 2017, a retrospective study looked back in time at hospital birth data from over 77,000 pregnant people aged 35 years and over (Knight et al. 2017). Everyone was giving birth for the first time between 2009 and 2014 at a National Health Service (NHS) hospital in England. They excluded people with pre-existing conditions (high blood pressure, diabetes, and heart or lung disease), multiple births, previous complicated births, and those with medical reasons for induction.
The study was conducted because of national guidelines recommending that induction be carried out between 41 and 42 weeks of pregnancy. The researchers wanted to know if routine induction at or after 39 weeks of pregnancy reduced the risk of perinatal death in first-time mothers aged 35 years or older compared with expectant management. Remember, the "35/39 Trial" was too small to tell if induction decreased the risk of stillbirth or newborn death.
The results? At 39 weeks, there was no difference in Cesareans or in-hospital perinatal death between people who were induced versus those who were expectantly managed. They did find less fetal hypoxia (oxygen deficiency) in labor and less meconium aspiration syndrome (when a baby inhales a mixture of meconium and amniotic fluid into the lungs at birth) with elective induction at 39 weeks.
However, the researchers found that induction at 40 weeks was linked to a lower risk of in-hospital perinatal death (0.08% versus 0.26%) compared to expectant management until 41-42 weeks. The absolute risk of perinatal death was still low; it would take around 562 inductions at 40 weeks to prevent 1 perinatal death. Induction at 40 weeks was linked to a slight increase in Cesareans and vaginal births with forceps/vacuum compared to expectant management at 40 weeks. Limitations of the study include the reliance on observational data; however, the study design was used because randomized trials are not always large enough to detect a difference in rare outcomes such as perinatal death (as we saw with the 35/39 Trial).
The authors concluded that changing the routine offer of induction from 41-42 weeks to 40 weeks in people 35 and older might reduce overall rates of perinatal death.
What is the evidence on elective induction in general?
In a 2020 Cochrane review and meta-analysis, researchers compared people who were electively induced at or beyond term versus those who followed expectant management (Middleton et al., 2020). They included 34 randomized, controlled trials with over 21,000 low-risk participants.
Most of the data came from trials of induction that took place at 41 weeks or later. The largest trials included were the ARRIVE trial (6,000+), the Hannah Post-Term trial (3,000+), and the Wennerholm trial (2,500+). Over half the participants were from trials published in the last seven years. Most trials were judged to be low to moderate risk of bias
The results? A policy of induction was linked to fewer perinatal deaths compared to expectant management, though absolute rates were small (0.4 versus 3 deaths per 1000, "high-certainty evidence"). Overall, the number needed to treat was 544 people with induction to prevent 1 perinatal death. Specifically, there were fewer stillbirths with a policy of induction (2 stillbirths out of 9,418 participants versus 16 out of 9,377).
A policy of induction was also linked to slightly fewer Cesareans compared to expectant management (16.4% versus 18.7%) and no difference in births with forceps or vacuum.
They were not able to find differences between timing of induction (< 40 versus 40-41 versus > 41 weeks), whether or not this was someone's first birth, or by the state of the cervix for perinatal death, stillbirth, or Cesarean.
The authors concluded that individualized counseling might help pregnant people choose between elective induction at or beyond term or expectant management, and that providers must honor their values and preferences. We need more research to know who would or would not benefit from elective induction and the optimal time for induction is still not clear from the research.
For more information on induction vs. expectant management in people of all ages, see the Evidence Based Birth® Signature Article on Inducing for Due Dates here.
Elective Cesarean
The ACOG Committee Opinion about Cesarean on maternal request estimates that 2.5% of all births in the U.S. are Cesareans on maternal request (ACOG, 2019). The Committee Opinion states, "In the absence of maternal or fetal indications for cesarean delivery, a plan for vaginal delivery is safe and appropriate and should be recommended." They do not list advanced maternal age as a medical indication for Cesarean.
Maternal request for Cesarean increases with advancing maternal age. Pregnant people aged 35 and older are about two times more likely to have a Cesarean upon maternal request compared to younger pregnant people (Lavecchia et al. 2016). This is concerning because planned Cesarean significantly increases the risk of severe complications in healthy pregnant people aged 35 and older.
It's not ethical to randomly assign healthy pregnant people to planned vaginal birth versus planned Cesarean, so we must rely on large observational studies. A large U.S. study evaluated maternal outcomes in over 442,000 healthy people aged 35 and older who gave birth from 2003 to 2012 (Lavecchia et al. 2016). About 8% of people in the cohort planned elective Cesareans.
When compared to the people who planned vaginal births, those who planned Cesareans had a significantly higher death rate (2.6 per 10,000 versus 0.4 per 10,000). People who had elective Cesareans also experienced more complications, including hysterectomy, cardiac arrest, acute kidney failure, and sepsis. The authors concluded, "When possible, planned cesarean deliveries should be avoided in this population."
To read more about the pros and cons of Cesarean, check out the Childbirth Connection resources here.
Birth in Midwifery-Led Settings
The Birthplace in England study was a large study looking at the birth outcomes of nearly 80,000 people who were at least 37 weeks along, planned a vaginal birth, and received care from a midwife during labor during the years 2008 to 2010. The researchers broke down that group further to compare outcomes for "older" people giving birth outside of a hospital setting.
For this particular analysis, researchers looked at about 63,000 people out of the total group— participants who were "low-risk" and between 37 weeks 0 days and 42 weeks and 0 days. Out of these, there were 12,078 people who were 35 or older, of whom 4,581 planned a birth at home, 1,923 planned a birth in a freestanding birth center, and 2,506 planned a birth in an "alongside midwifery unit" (midwifery birth center located in the hospital, but separate from labor and delivery). The rest planned a birth in a traditional hospital labor and delivery unit.
To watch a video tour of an alongside midwifery unit in the United Kingdom, click here (http://bit. ly/2pTBN2E).
All of the midwifery-led locations (home, birth center, and alongside midwifery units) were combined and compared as a whole to the traditional hospital labor and delivery (obstetric) environment. For the purposes of this section, we will refer to home, birth center, and alongside midwifery unit group as "midwifery-led," since that is common to the three groups.
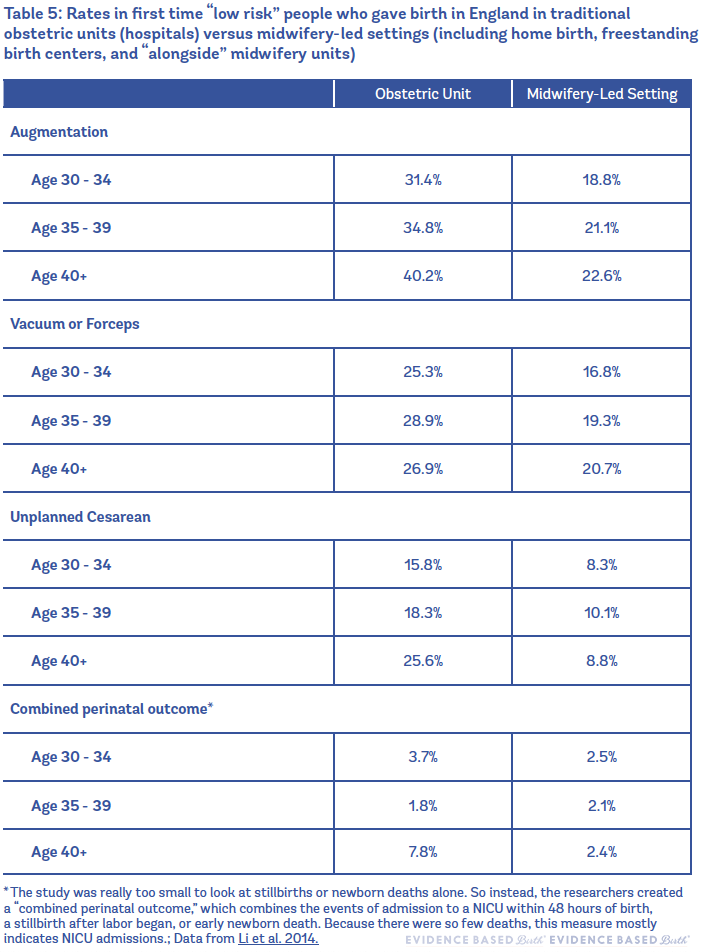
The researchers found that older parents had similar newborn outcomes whether they gave birth in a midwifery-led setting or inside a hospital labor and delivery unit, while those giving birth in a midwifery-led setting outside the traditional labor and delivery (obstetric) unit had fewer interventions (Li et al. 2014). That included lower rates of augmentation, vacuum/ forceps, and fewer adverse outcomes requiring an obstetrician's intervention, such as unplanned Cesareans and 3rd or 4th degree tears.
As an example, 42% of first-time mothers age 35 to 39 had Pitocin® augmentation in a planned hospital birth, while only 23% of first-time mothers the same age had Pitocin® in a planned midwifery-led setting. Similarly, only 5% of birthing people 40 or older had a 3rd or 4th degree tear in a midwifery-led setting, compared to 11% of similarly aged people in an obstetric unit.
Table 5 shows rates in first-time "low-risk" people who gave birth in England in traditional obstetric units (hospitals) versus midwifery-led settings (including home birth, freestanding birth centers, and "alongside" midwifery units).
There were more perinatal outcome events in the 40+ age group who gave birth in a hospital labor and delivery unit, which were probably related to higher rates of NICU admissions in this group.
Another study that used data from the Midwives Alliance of North America Statistics Project (MANA Stats) examined outcomes from nearly 50,000 midwife-attended, planned community births in the home or freestanding birth center setting (Bovbjerg et al. 2017). Pregnant people aged 35 and older made up 19% of participants in the dataset.
The researchers found that older pregnant people who planned community birth and did not have additional risk factors were not at higher risk for childbirth complications. There was no evidence of increased risks of perinatal death or postpartum hemorrhage with older maternal age. For most outcomes, older pregnant people had absolute risks only a percentage point or two higher than younger people. The authors concluded that community birth with advanced maternal age, in the absence of other risk factors, is not as risky as previously assumed.
What about fetal testing?
Because of fears about stillbirth, many providers recommend that people 35 years and older have fetal monitoring or testing towards the end of pregnancy, in the hopes of identifying any babies that are at risk for stillbirth.
In their chart review, Fox et al. (2013) compared 1,541 pregnant people who were 35 and older to 2,928 pregnant people who were younger than 35. The standard in their practice for clients 35 and older is to perform the ultrasound part of a biophysical profile (BPP) test weekly, starting at 36 weeks, and to plan birth no later than 41 weeks (Fox et al. 2013).
The biophysical profile is a 5-part test that was first used in the year 1980. It consists of a nonstress test and an ultrasound that looks at large fetal movements, fetal muscle tone, fetal breathing, and amniotic fluid volume. The BPP is scored out of 10 points, with 2 points awarded for presence of each feature, and 0 for the absence. A score of 8 or 10 is normal, a score of 6 is questionable and calls for further testing or delivery, and a score of 4, 2, or 0 is abnormal and usually calls for immediate birth. Many providers today do not perform a nonstress test if the ultrasound part of the BPP is normal; it is thought that the nonstress test does not add any predictive power to the BPP (Lalor et al. 2008).
The researchers found there was no statistical difference in the rate of stillbirth between older and younger groups. In both older and younger groups, the rate of stillbirth at ≥20 weeks was 3.4 to 3.9 per 1000, and the rate of stillbirth at ≥36 weeks was 1.1 to 1.4 per 1000. Pregnant people who were 35 and older had a higher rate of induction (18.5% vs. 15.8%), and gave birth 3 days earlier, on average (38.4 weeks vs. 38.9 weeks). The authors did not report on Cesarean rates.
The authors of this study argued that their policy of routine BPP tests starting at 36 weeks and a policy of planning birth (by induction or Cesarean, if necessary) no later than 41 weeks brought the stillbirth rate of people 35 and older down to the same rate as younger people. However, because of the study design (using a chart review to look backwards in time), we cannot say with any certainty if their fetal monitoring was the cause of the low stillbirth rates.
A retrospective study at a single U.S. hospital evaluated Cesarean and induction rates after introducing routine antenatal testing at their institution (Levine et al. 2015). They compared pregnant people 40 years of age and older who gave birth before versus after implementing the testing policy. The testing consisted of twice-weekly nonstress tests and twice-weekly amniotic fluid assessment beginning at 32 weeks. A total of 276 pregnant people were included (147 who gave birth before the policy and 129 who gave birth after the policy).
The researchers found no difference in the induction rate between groups. There was a higher overall Cesarean rate after the testing policy (53% versus 39%); however, after they excluded people with prior Cesareans, there was no difference in the risk of primary Cesarean. The authors state the study may not have been large enough to detect a difference in primary Cesareans. So, at this time, there is still very little evidence on how fetal testing impacts induction and Cesarean rates.
In another paper published by Fretts et al. (2004), researchers used a mathematical model to try and estimate the potential effects of fetal monitoring. Their math analysis was based on stillbirth rates collected by McGill University in Canada. The results from Fretts et al.'s math model suggested that if first-time mothers 35 and older had weekly fetal monitoring starting at 37 weeks, and were induced by 41 weeks, that it would take 863 fetal tests, 71 inductions, and 14 Cesareans to avoid 1 stillbirth (Fretts et al. 2004).
What do the guidelines say?
A new systematic review of clinical guidelines on female age-related fertility decline found 19 guidelines, 11 of which they considered to be high quality (Trawick et al. 2021). However, even among the high quality guidelines, there was little agreement about key issues. For example, guidelines stated conflicting ages at which female fertility declines, ranging from 30 to "late 30s." Nearly half (5/11) of the guidelines considered to be high quality did not even discuss pregnancy or childbirth risks related to advanced maternal age. The review authors concluded that existing guidance on age-related fertility decline is "inconsistent and incomplete." Better clinical practice guidelines are needed to inform counseling so that people considering pregnancy can make informed reproductive choices.
In 2012, the Society for Maternal and Fetal Medicine published an opinion that encouraged pregnant people and care providers to weigh the risks and benefits to decide whether to perform fetal testing or electively induce labor in mothers 35 and older. They also recommend that when mother and baby are healthy, induction should not take place before 39 weeks. They state that there is insufficient evidence to show that fetal testing for advanced maternal age can reduce the risk of stillbirth, and that care providers and pregnant people should weigh the pros and cons of such testing. To read the opinion statement, click here.
Neither ACOG nor ACNM (American College of Nurse Midwives) give official recommendations about caring for pregnant people who are 35 and older. ACOG published Frequently Asked Questions in 2020 on having a baby after age 35 (ACOG, 2020). The FAQS address how aging affects fertility and pregnancy risks. ACOG mentions older maternal age as one of the risk factors for stillbirth in their FAQs and in an Obstetric Care Consensus on the Management of Stillbirth (ACOG/SMFM, 2020).
The Royal College of Obstetricians and Gynecologists (RCOG) published a scientific paper in 2013 about AMA that you can read here. In their paper, RCOG does not make specific recommendations, but they do state that "Women ≥ 40 years of age having a similar stillbirth risk at 39 weeks of gestation to women in their mid-20s at 41 weeks of gestation, at which stage the consensus is that induction of labour should be offered to prevent late stillbirth."
In 2017, RCOG responded to the Knight et al. study with their position on the study's findings. The President of RCOG said:
"This large study supports emerging research which suggests that moving forward the offer of induction to 40 weeks may play a role in reducing the proportion of stillbirths and neonatal deaths, but it should be emphasised that this study is the first to focus on a specific patient group – first time mothers aged 35 and over…The study finds a significant reduction in the risk of perinatal death, but the absolute risk is still low even among older mothers…While induction is safe and studies have shown no short-term adverse impact on mother or baby, induction of labour represents an intervention, is associated with costs to the service, and can be a more prolonged process than spontaneous labour. These findings should help women over the age of 35 make an informed choice with regards to induction of labour and are likely to influence the production of future guidelines."
The 2019 UpToDate recommendation for pregnant people aged 40 and older is to give birth at 39 weeks. This recommendation is based on Grade 2C evidence. UpToDate states that Grade 2C is essentially an expert opinion; a weak recommendation based on low-quality evidence.
A systematic review of clinical guidelines on induction found mixed recommendations on induction for maternal age—likely due to the limited and conflicting evidence on the topic (Coates et al. 2019). Most guidelines state that people 40 years of age and older may be offered an induction. Of the guidelines that recommend induction for advanced maternal age, recommendations vary about whether it should occur at 39 weeks, between 39 and 40 weeks, or at 40 weeks. One guideline (from Australia) states that maternal age alone is not an acceptable indication for induction. They base this conclusion on the findings of the "35/39 Trial" discussed above.
What Age Can U Take Birth Control
Source: https://evidencebasedbirth.com/advanced-maternal-age/
0 Response to "What Age Can U Take Birth Control"
Post a Comment